Multi-photon microscopy with fiber lasers
Femtosecond fiber lasers for simplifying microscopy
Non-linear microscopic techniques, such as 2-photon fluorescence microscopy or second harmonic generation (SHG) microscopy, have become key technologies in biological imaging, enabling three-dimensional, non-invasive studies of tissue with sub-cellular resolution on the micrometer scale. In contrast to conventional linear fluorescence microscopy, the nonlinear character and the longer excitation wavelengths offer key advantages of larger probing depths and lower phototoxicity.
Interestingly, the two-photon absorption cross section of typical fluorescent proteins and dyes has relatively large spectral bandwidths of > 100 nm. Thus, almost all commonly used fluorophores can be excited by just three single-wavelength lasers, operating at 780 nm, 920 nm, and 1050 nm. Consequently, the microscopy community has increasingly shifted over the last few years towards fixed-wavelength mode-locked femtosecond fiber lasers at 780 nm, 920 nm or 1050 nm as a cost-efficient and easy-to-use alternative for multi-photon microscopy applications.
TOPTICA's offer
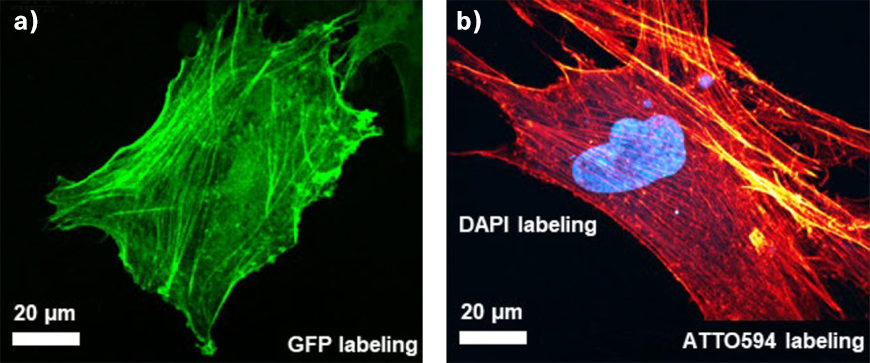
TOPTICA’s femtosecond fiber lasers are easy to use and provide optimal performance. In particular, TOPTICA’s FemtoFiber ultra lasers are designed to be ideal for imaging green (GFP), red (RFP), blue (BFP) fluorescent proteins, auto-fluorescence, or SHG signals to address highly-relevant biological questions. It is no question why these laser systems are already widely adopted in non-linear microscopes worldwide.
To achieve the best image quality, TOPTICA’s FemtoFiber ultra is equipped with Clean Pulse Technology that reduces pedestals and side-wings on the temporal pulse profile. Thus, the full laser power contributes to two-photon excitation. Clean Pulse Technology provides the highest fluorescence image brightness and contrast while thermal heating of the sample is kept to a minimum.
TOPTICA’s FemtoFiber ultra also streamlines usability: The laser includes beam conditioning to adapt the input laser beam parameters to the microscope. Firstly, software-control of the group delay dispersion (GDD) pre-compensation enables user-friendly and repeatable optimization of fluorescence signal strength. Secondly, an integrated acousto-optic modulator (AOM) enables fast power-modulation and flyback blanking in synchronization with the microscope’s beam scanner. This minimizes sample damage and photo-bleaching. Thirdly, the laser heads are extremely compact and passively-cooled, offering much lower operating and maintenance costs to competing technologies.
Additionally, the FemtoFiber ultra 920 and 1050 can now be equipped with femtosecond fiber delivery, optimized to deliver high quality pulses with new fiber technology. This opens new possibilities for integration into modern microscopy set-ups. By simplifying complex free-space pulse delivery on an optical table to a simple FC/APC fiber connection, our solution further boosts usability while still providing high performance for the best fluorescence image quality.