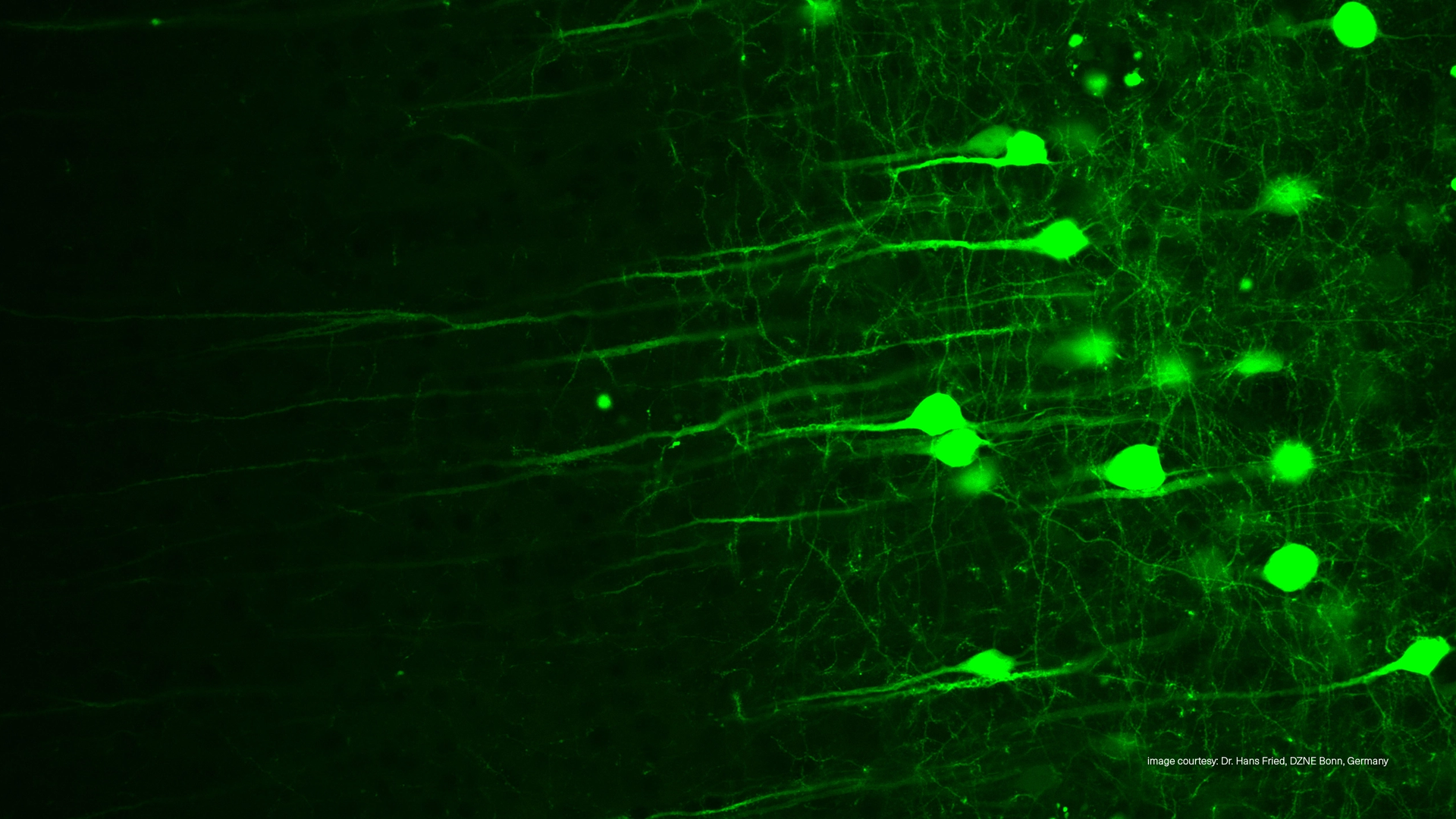
Two-Photon Microscopy (TPEF)
Seeing deeper, clearer, gentler
The most fascinating biological processes often happen deep inside living tissue, which light normally cannot reach due to scattering and absorption. Two-Photon Excitation Fluorescence Microscopy (TPEF) breaks this barrier, allowing scientists to image intact organisms, live cells, and even neuronal networks hundreds of micrometers beneath the surface — without damage or distortion.
Two-photon microscopy combines the precision of laser scanning with the power of nonlinear optics to visualize with sub-cellular resolution in depth, in 3D, and in real-tme.
And at the heart of this revolutionary imaging technique lies the femtosecond laser — delivering pulses of light ultra-short and intense enough to excite fluorescence with two photons at once.
The principle: Two photons, one excitation
In conventional (single-photon) fluorescence microscopy, a fluorophore absorbs a single high-energy photon to reach its excited state.
In two-photon microscopy, the same excitation occurs through the simultaneous absorption of two photons of lower energy — typically in the near-infrared (NIR) region.
The likelihood of two-photon absorption is intensity dependent and it can only happen at the focal point of a tightly focused femtosecond laser beam.
As a result:
- Excitation is confined to a tiny focal volume, eliminating the need for a pinhole or confocal aperture in the detection scheme.
- Photobleaching and photodamage outside the focus are drastically reduced.
- Scattering is minimized, allowing imaging much deeper into biological tissue.
By scanning the focal point in three dimensions, researchers obtain high-resolution, optically sectioned images — just like in confocal microscopy, but with far greater depth and gentleness.
Why two-photon microscopy matters
Two-photon microscopy has transformed biophotonics, neuroscience, and clinical imaging, offering a unique combination of optical sectioning, deep tissue penetration, and live-cell compatibility.
Its key advantages include:
- Deeper penetration depth (up to 1 mm or even more)
- Reduced phototoxicity and photobleaching, ideal for live tissue imaging.
- No pinhole required — intrinsic optical sectioning from nonlinear excitation.
- Better signal-to-noise ratio in scattering tissue, even at large depths.
- Simultaneous multi-color excitation of different dyes or auto-fluorescence using a single femtosecond source.
For these reasons, TPEF is now a standard technique in neuroscience, developmental biology, and intravital imaging — enabling researchers to study live systems as they function in real time.
Typical biological samples in two-photon imaging
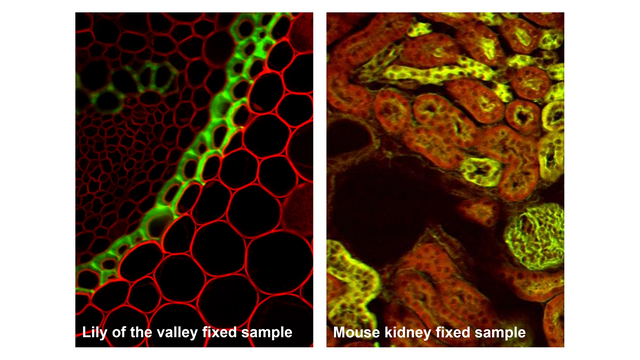
Two-photon microscopy excels at visualizing thick, living, or scattering samples that are challenging for conventional microscopy:
- Brain tissue – in vivo imaging of neuronal activity and synaptic dynamics.
- Zebrafish, Drosophila, or C. elegans embryos – developmental biology in transparent or semi-transparent organisms.
- Organoids and tissue explants – 3D model systems for organ development or disease.
- Vasculature and tumor microenvironments – blood flow, angiogenesis, and drug delivery.
- Skin and eye tissue – non-invasive optical biopsy and clinical imaging.
The ability to visualize structures hundreds of micrometers deep in living samples has made two-photon microscopy indispensable for studying how cells, tissues, and networks behave in their natural context.
The power of femtosecond lasers
To drive the nonlinear two-photon excitation process efficiently, the laser must deliver:
- High peak power (requiring typically femtosecond pulses).
- Diffraction-limited beam quality.
- Wavelength matching to the fluorophore’s absorption.
- A repetition rate matched to the fluorecent lifetime of the dye (typically 20-80 MHz)
Femtosecond fiber lasers have become the preferred light source for TPEF due to their stability, compactness, and hands-free operation — outperforming traditional Ti:Sapphire lasers in reliability and maintenance effort.
Recommended fixed wavelengths and fluorophores
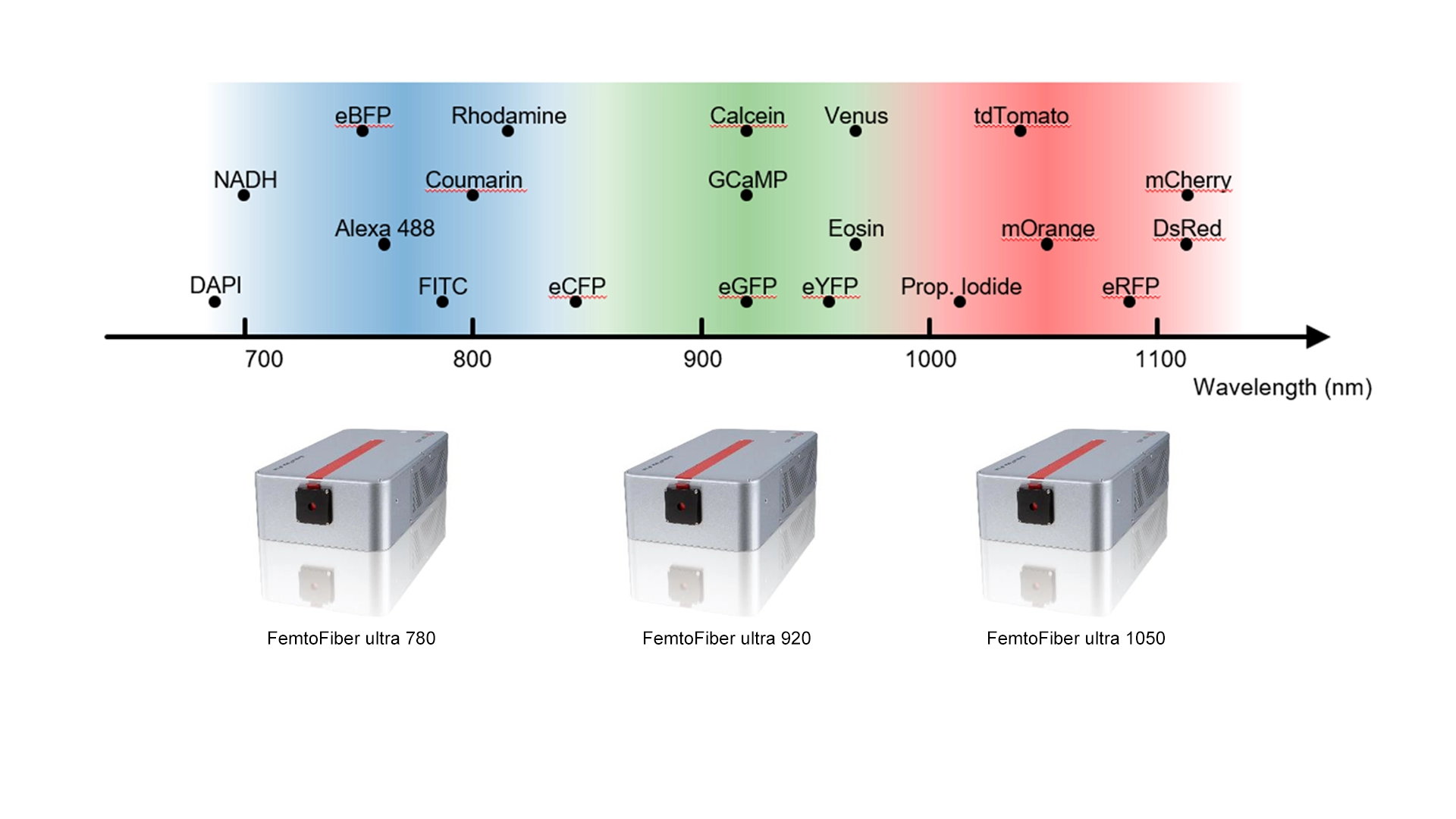
Different fluorophores and fluorescent proteins require different excitation wavelengths.
Fixed-wavelength femtosecond fiber lasers at 780 nm, 920 nm, and 1050 nm cover most common dyes or auto-fluorescent used probes in two-photon imaging:
| Laser Wavelength | Typical Fluorophores / Chromophores | Application Examples |
|---|---|---|
| 780 nm | DAPI, Alexa Fluor 350, Fura-2, NADH | Calcium imaging, DNA staining, metabolic imaging |
| 920 nm | GFP, Alexa Fluor 488, Oregon Green, GCaMP, Eosin, CFP, FAD | Neuronal calcium imaging, cell tracking, metabolic imaging |
| 1050 nm | tdTomato, mCherry, Alexa 594, Rhodamine, RFP, mOrange | Deep tissue red fluorescence imaging, dual-color imaging |
By selecting the appropriate wavelength, researchers can maximize signal strength, minimize tissue heating, and achieve optimal penetration depth for their specific application.
Fiber lasers – Simplicity meets stability
TOPTICA’s femtosecond fiber lasers combine fixed, biologically relevant wavelengths with robust fiber architecture — providing plug-and-play operation for demanding research and clinical environments.
Compared to bulky, alignment-sensitive Ti:Sapphire or OPO systems, TOPTICA's femtosecond fiber lasers offer:
- Clean Pulse Technology for highest fluorescence image brightness.
- Software-control of the group delay dispersion (GDD) pre-compensation
- An integrated acousto-optic modulator (AOM).
- Extremely compact and passively-cooled.
- Seamless integration through optional fiber delivery with COOLAC.
- Fast, hands-off startup and no user alignment.
- Consistent performance across months of operation.
- No noise or vibration (due to passive air cooling)
The laser systems with fiber delivery route the laser beam directly into the microscope via polarization-maintaining hollow-core fibers. This drastically simplifies experimental setups and minimizes drift or vibration sensitivity. With TOPTICA's COOLAC the lasers offer hands-off, automated fiber coupling that eliminates manual alignment at installation, optimizes fiber coupling at the touch of a button, and monitors fiber coupling efficiency completely internally without the need for external tools or equipment. Especially, miniaturized two-photon microscopes like Mini2P, benefit from this laser technology.
Simplifying the path to clinical two-photon imaging
Until recently, the complexity and cost of traditional ultrafast laser systems limited two-photon microscopy to specialized research labs.
Fiber-based femtosecond lasers are changing that paradigm — combining turnkey operation and robustness suitable for clinical and translational environments.
This simplification is paving the way for clinical-grade two-photon imaging systems in dermatology, ophthalmology, histology and endoscopy.
Emerging Clinical Examples
- In vivo skin microscopy: Non-invasive imaging of collagen, elastin, and melanin structures in patients. Kann man hier direkt das EnspectraHealth paper verlinken?? Scientific Publication: K. Montgomery, et al., Handheld multiphoton and pinhole-free reflectance confocal microscopy enables noninvasive, real-time cross-sectional imaging in skin; Nature (2024)
- Ophthalmic diagnostics: High-resolution imaging of retinal layers using near-infrared excitation
- Label-free pathology: Two-photon autofluorescence combined with SHG (Second Harmonic Generation) for real-time tissue characterization.
The new generation of compact, fiber-coupled femtosecond lasers brings the promise of two-photon microscopy from the research bench to the clinical applications.
Scientific References
-
Multimodal optical coherence tomography and two-photon light sheet fluorescence microscopy for embryo imaging
Md Mobarak Karim et al, “Multimodal optical coherence tomography and two-photon light sheet fluorescence microscopy for embryo imaging”, Journal of Biomedical Optics, Vol. 30, Issue 6, 060501 (June 2025)
-
Influence of laser pulse shape and cleanliness on two-photon microscopy
Shau Poh Chong and Peter Török, "Influence of laser pulse shape and cleanliness on two-photon microscopy," Opt. Continuum 3, 552-564 (2024)
-
Femtosecond fiber delivery at 920 nm for two-photon microscopy
Konrad Birkmeier, et al., "Femtosecond fiber delivery at 920 nm for two-photon microscopy", Proc. SPIE 12847, Multiphoton Microscopy in the Biomedical Sciences XXIV, 1284703 (12 March 2024)
-
Evaluation of compact pulsed lasers for two-photon microscopy using a simple method for measuring two-photon excitation efficiency
Samir Saidi, Matthew Shtrahman, “Evaluation of compact pulsed lasers for two-photon microscopy using a simple method for measuring two-photon excitation efficiency“, Neurophotonics, Vol. 10, Issue 4, 044303 (November 2023)
-
Large-scale two-photon calcium imaging in freely moving mice
W. Zong, et al., Large-scale two-photon calcium imaging in freely moving mice, Cell https://doi.org/10.1016/j.cell.2022.02.017 (2022)
-
High energy (>40 nJ), sub-100 fs, 950 nm laser for two-photon microscopy
Ruihong Dai , et al., High energy (>40 nJ), sub-100 fs, 950 nm laser for two-photon microscopy, Optics Express, Vol. 29, Issue 24 (2021)
-
Robust functional imaging of taste sensation with a Bessel beam
J. Han, et.al., Robust functional imaging of taste sensation with a Bessel beam, Biomedical Optics Express 12, 5855 (2021)
-
Simplifying two-photon microscopy (2020)
Simplifying two-photon microscopy (2020)
-
Next generation two-photon microscopy using the FemtoFiber ultra 920 fiber laser
Dr. Max Eisele, Bernhard Wolfring "Next generation two-photon microscopy using the FemtoFiber ultra 920 fiber laser" (2019)
TOPTICA Photonics:
Bringing the light that lets us see deeper - into life, into function, into future.